SAN CLEMENTE, June 28, 2022 — Reflow Medical, Inc., a California-based medical device company, has completed enrollment in the DEEPER LIMUS clinical trial (NCT04162418) to evaluate the Temporary Spur Stent System, a patented device designed to treat long, diffuse and severely calcified infrapopliteal disease. The system allows for uniform expansion of the stent to maximize lumen diameter and may reduce acute vessel recoil and dissections.
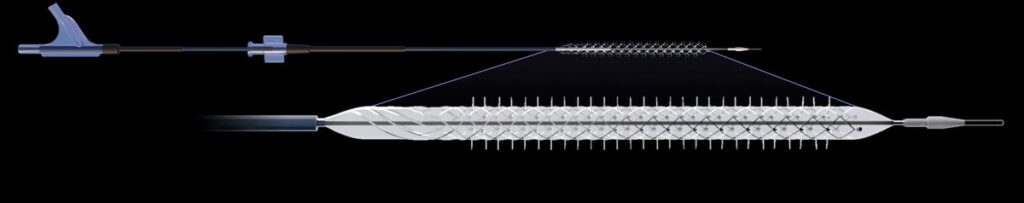
Engineered to fulfill an unmet clinical need in treating patients suffering from Critical Limb Ischemia (CLI), the Temporary Spur Stent System consists of a unique, retrievable nitinol stent system with radially expandable spikes or “spurs” designed to create channels for increased uptake of antiproliferative drugs (“limus” based) into the vessel wall. The system is then recaptured and removed, without leaving anything behind.
“We were impressed from the start with the acute luminal gain we were able to achieve,” says Marianne Brodmann, MD, who is a Professor and Vascular Specialist at the Medical University of Graz, Austria, and the Principal Investigator for the study. “It’s eye-opening, how the vessel responds. The device is also intuitive and easy to use.”
Teo Jimenez, Reflow Medical’s Senior Vice President of Research and Development said, “Finishing enrollment in this study continues to highlight the versatility and safety of the Spur device with multiple drug-coated platforms.”
“On behalf of the team here at Reflow, we offer sincere thanks and gratitude for the partnership with Professor Brodmann and her team,” commented Isa Rizk, CEO and Co-founder of Reflow Medical. “Their ability to perform this study during a global pandemic and achieve this milestone shows their commitment and dedication to advancing technologies in the CLI space.”
About Reflow Medical, Inc.
Reflow Medical, Inc. focuses on empowering physicians through the design and development of innovative and effective technologies for cardiovascular disease. The Reflow product portfolio includes products used to treat cardiovascular disease in the peripheral vasculature as well as the coronary arteries.
Contact: Jennifer Carlyle
jcarlyle@reflowmedical.com
949-481-0399
