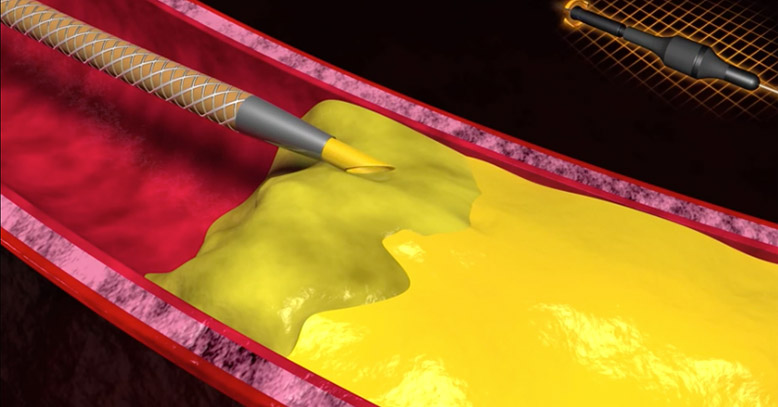
SIMPLE & EFFECTIVE
Expanded indication to Cross Chronic Total

View a video demonstration
the Wingman™
Compatible with the physician’s choice of guidewire. Works with the Reflow Spex™ and SpexLP™ catheters for added support in lesion crossing.
RAPID, ROUTINE LESION CROSSING “ON-THE-FLY”
- Compatible with physician-preferred guidewire and procedural technique.
- Keeps the physician in control of the device advancement and activation at all times.
- Provides a similar safety profile to conventional support catheters.
- Eliminates the need for expensive, complicated energy devices to cross most lesions.
- .014”, .018” and .035” options allow treatment of a broad range of vasculature: SFA, iliac and below-the-knee.
- Reduces total procedure time.
- .014″C enables treatment of coronary vasculature.

BRAID-REINFORCED
HYPOTUBE MICROCATHETER
Provides added column strength and traction to resist kinks
CONTROL POINT™
ACTIVATION AND
ENGAGEMENT HANDLE
Allows you to simultaneously “push and twist” the beveled tip to anchor and penetrate the lesion
UNIQUE EXTENDABLE
BEVELED TIP
Improves tracking through extremely tight occlusions and difficult-to-cross vasculature
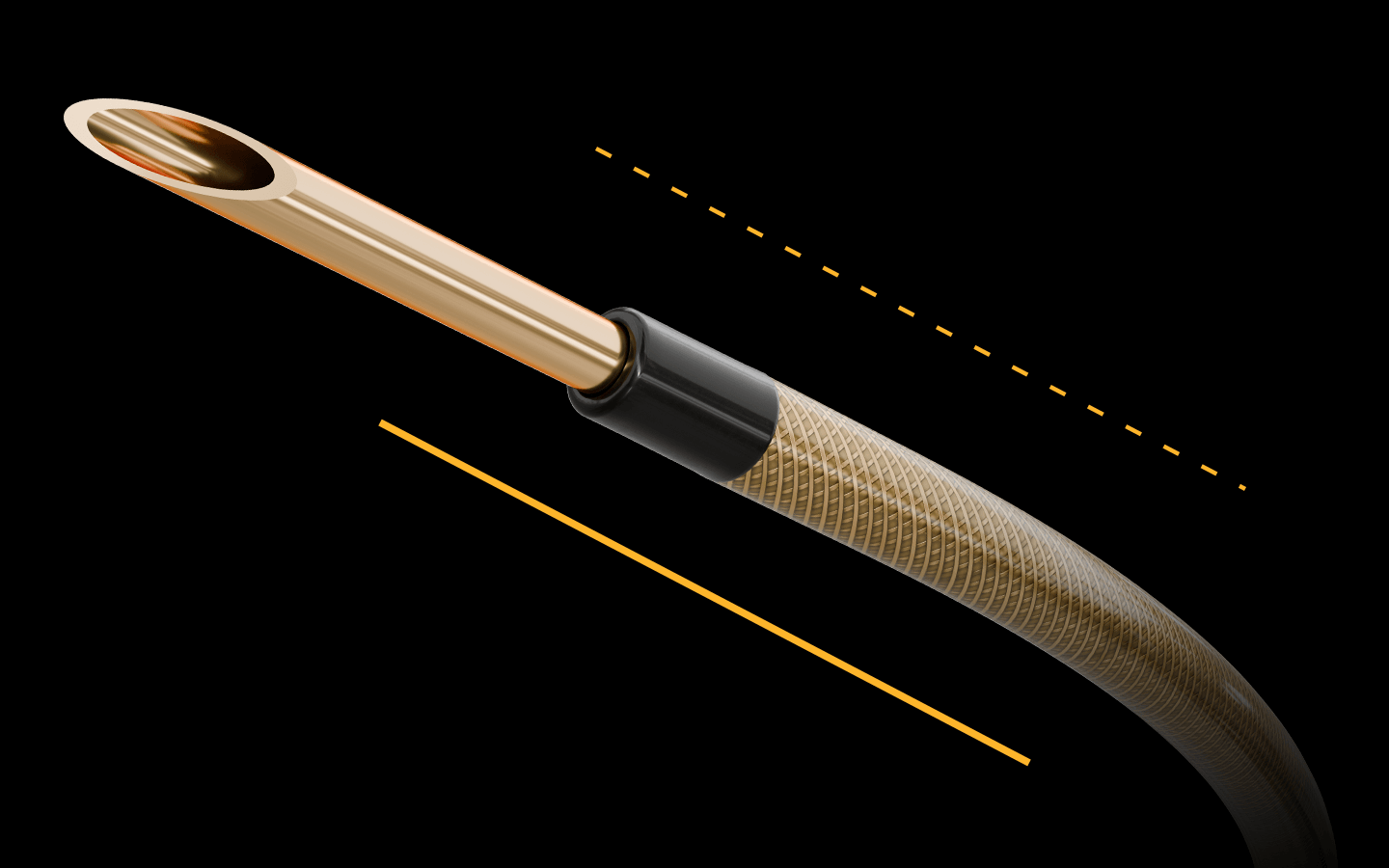
CONTROL COAT™
HYDROPHILIC
CATHETER COATING
Increases lubricity without compromising tactile feedback
©2024 Reflow Medical, Inc. All rights reserved. Reflow Medical, Wingman, and The Pulse of Medical Ingenuity are registered trademarks or trademarks of Reflow Medical, Inc. Federal law (USA) restricts these devices to sale by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the Indications, Contraindications, Warnings, Precautions, Complications, and Directions for Use.
Indications, Safety, & Warnings
CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the Indications, Contraindications, Warnings, Precautions, Complications, and Directions for Use.
Indications for Use
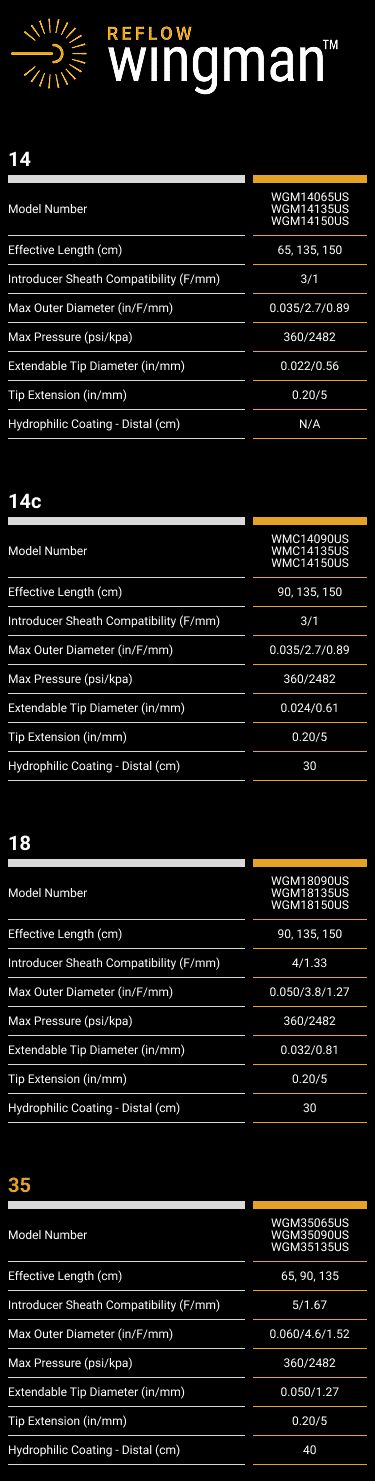
The catheters in the Wingman Family of Crossing Catheters (14/14C/18/35 – see specification table above) are intended to be used in conjunction with steerable guidewires to access discrete regions of the peripheral vasculature. Any one of these catheters may be used to facilitate placement and exchange of guidewires and other interventional devices, including facilitation of the intraluminal placement of diagnostic/interventional devices beyond peripheral stenotic lesions (including chronic total occlusions [CTOs]) and provide a conduit for delivery of saline solutions or diagnostic/therapeutic agents.
The Wingman 14C Crossing Catheters are also intended to be used in conjunction with steerable guidewires to access discrete regions of the coronary vasculature. In that area, they may also be used to facilitate placement and exchange of guidewires and other interventional devices and provide a conduit for delivery of saline solutions or diagnostic/therapeutic agents.
Contraindications
The Wingman 14, 18, & 35 Crossing Catheters are contraindicated for use in the coronary and cerebral vasculature. The Wingman 14C Crossing Catheters are contraindicated for use in the cerebral vasculature.
Warnings
Hydrophilic wires prone to excessive swelling (e.g., ZipWire) should not be used with Wingman Crossing Catheters.
Single Use only. Do not reuse/resterilize. Reusing the device could result in compromised device performance, cross-infection, and other safety related hazards.
Do not use if device is open or packaging is damaged.
Never advance, withdraw, or rotate an intravascular device against resistance until the cause is determined by fluoroscopy.
This device contains nickel and should not be used in patients with known allergies to nickel.
Precautions
Store in a cool, dry place. Protect from direct sunlight and high temperatures.
Use only appropriately sized ancillary device, as shown in the Specifications below.
Maximum Infusion Pressure: 360 psi (2482 kpa)
Use the catheter prior to the “Use By” date specified on the package.
The catheter should only be used by physicians qualified to perform percutaneous, vascular interventions.
Precautions to prevent or reduce clotting should be taken when any catheter is used in the vascular system. Use of systemic heparinization and heparinized saline solution should be considered.
Exercise care while handling the catheter during procedure to reduce the possibly of accidental damage, kinking or bending.
Manipulation of the catheter should only occur under fluoroscopy.
Complications
Vascular catheterization and/or vascular intervention may result in complications including but not limited to:
• Vessel dissection, perforation, rupture, or total occlusion
• Infection
• Hematoma
• Unstable angina
• Embolism
• Hypo/hypertension
• Acute myocardial infarction
• Arrhythmia, including ventricular fibrillation
• Death
If the catheter is damaged, this product may cut into a blood vessel wall. Extreme caution needs to be taken when removing a damaged device. In the case of complications resulting from the removal of the entire system, stop immediately the procedure, and perform appropriate treatment at the discretion of the physician.
©2024 Reflow Medical, Inc. All rights reserved. Reflow Medical, Wingman, and The Pulse of Medical Ingenuity are registered trademarks or trademarks of Reflow Medical, Inc. Federal law (USA) restricts these devices to sale by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the Indications, Contraindications, Warnings, Precautions, Complications, and Directions for Use.
QUESTIONS?
TALK TO A REFLOW PRO.
For more information, please send us your question or request today.
A Reflow pro will get back to you.