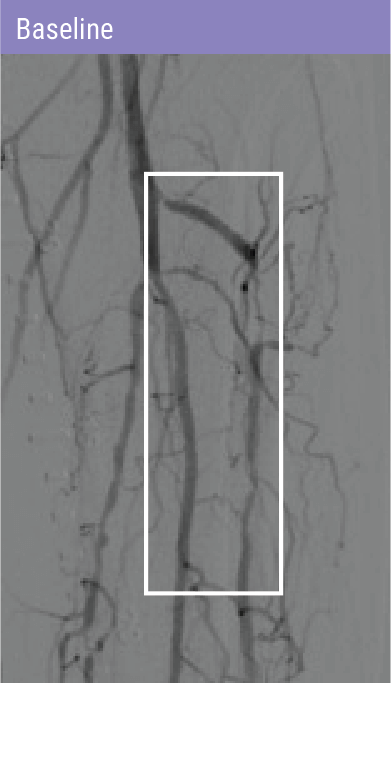
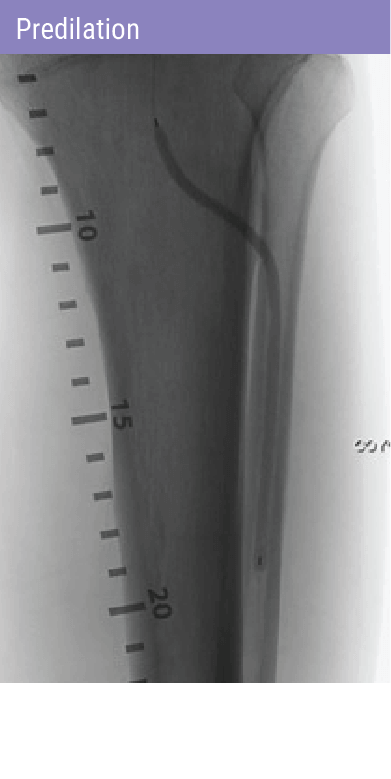
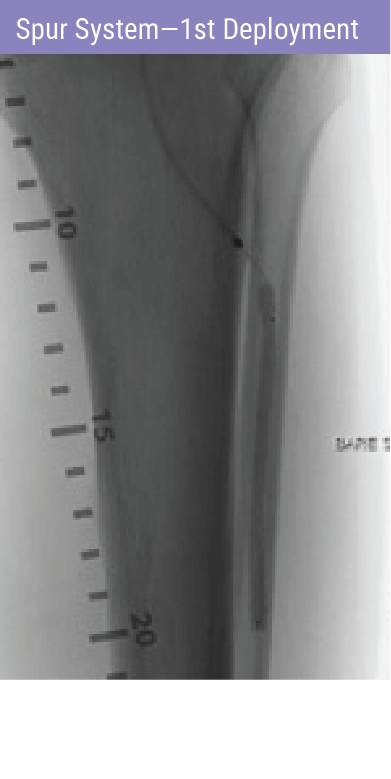
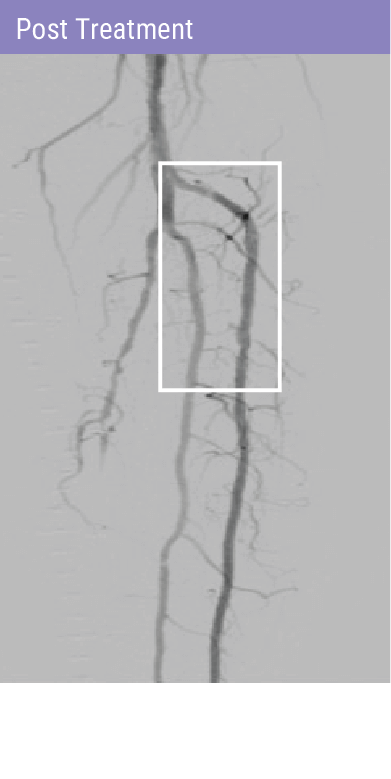
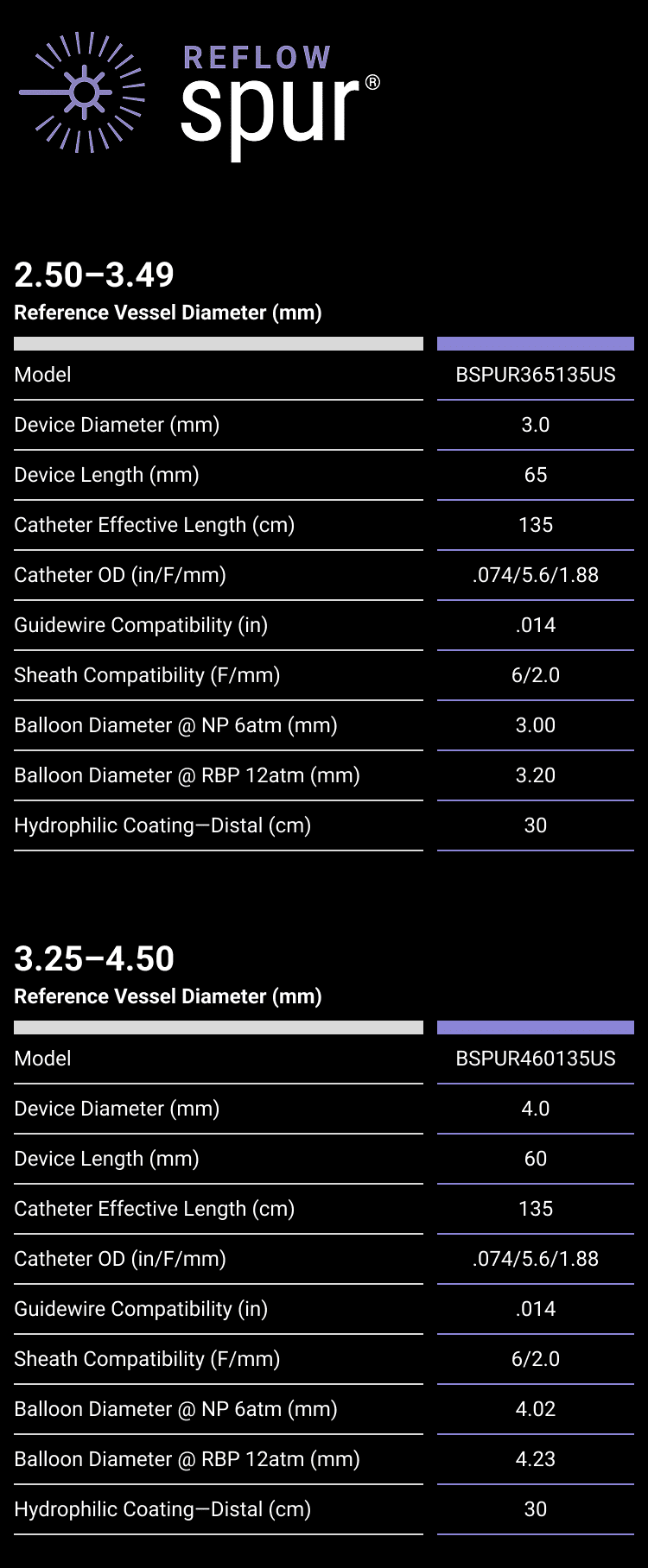
REVOLUTIONARY DEVICE FOLLOWING PREDILATATION FOR PATIENTS WITH BTK DISEASE

SPUR RETRIEVABLE SCAFFOLD THERAPY (RST)
A self-expanding stent with integrated dilation balloon catheter on an over-the-wire (OTW) system, designed for controlled penetration and lesion treatment after predilatation through a series of radially expandable spikes. Spur penetrates lesion to increase acute luminal diameter and modify the lesion morphology to change vessel compliance and reduce vessel recoil effect.
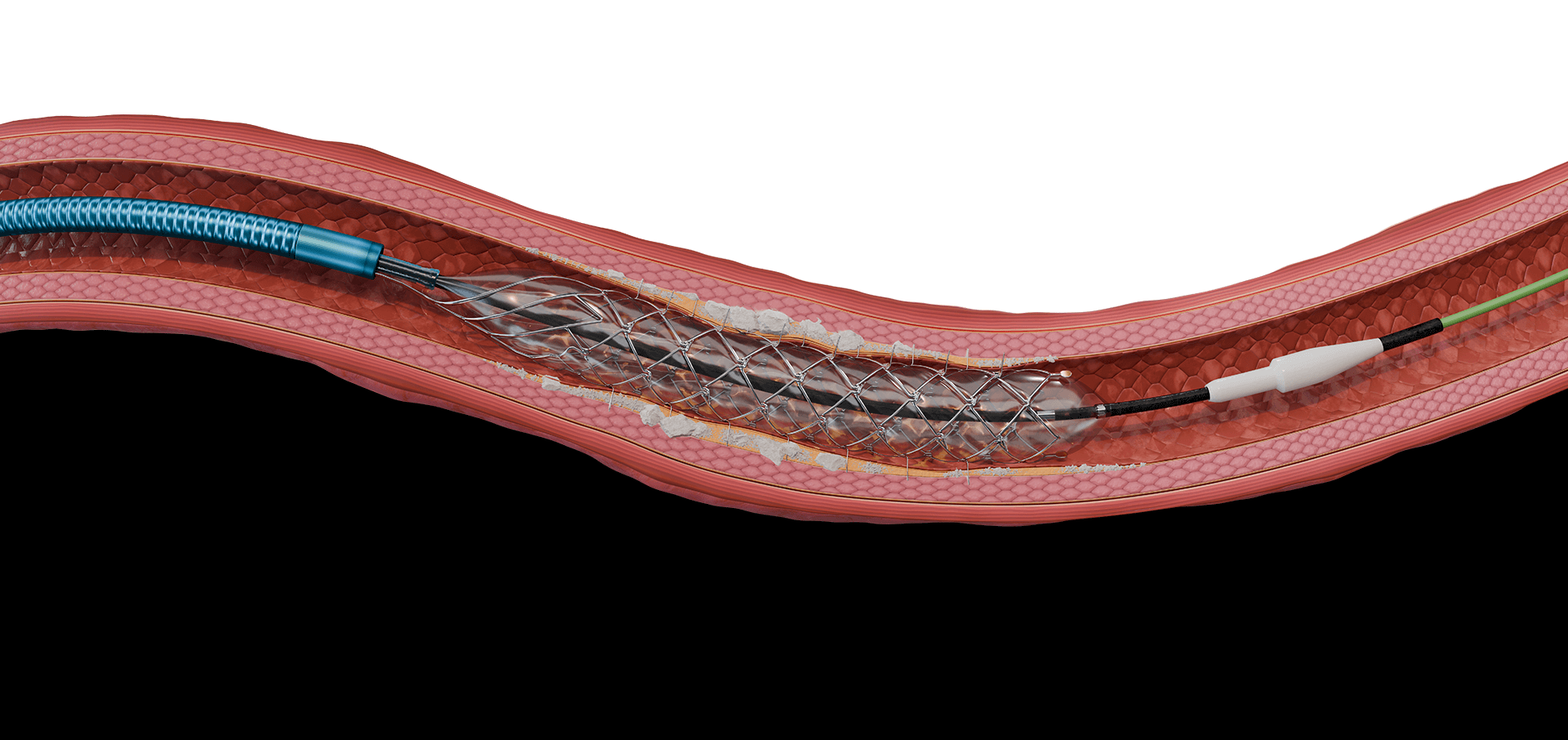
5.6F retractable shaft
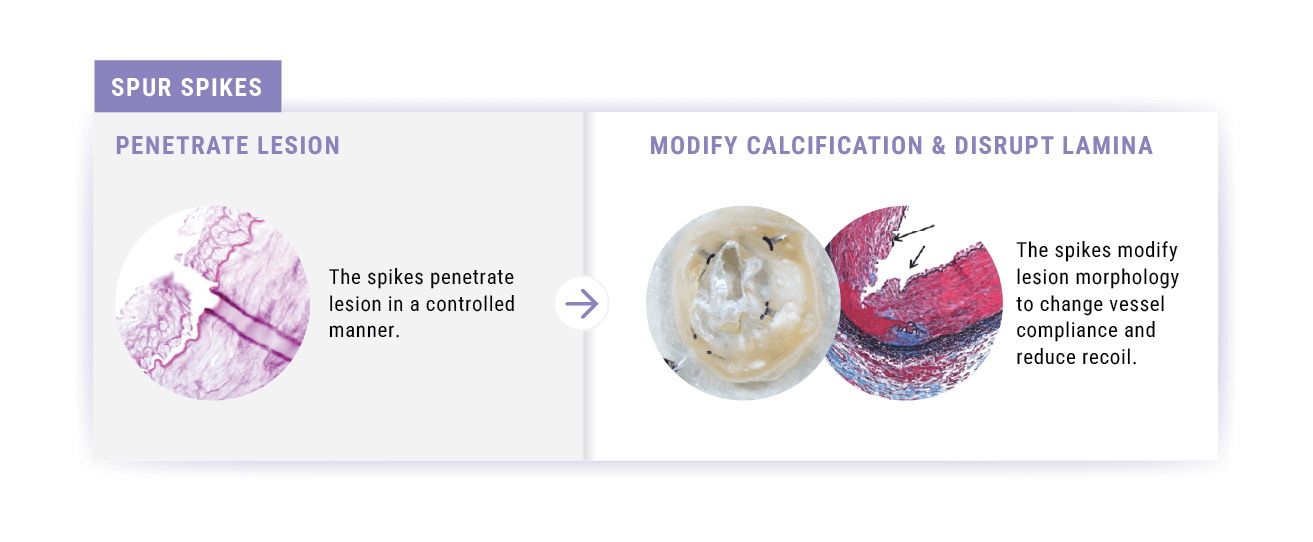
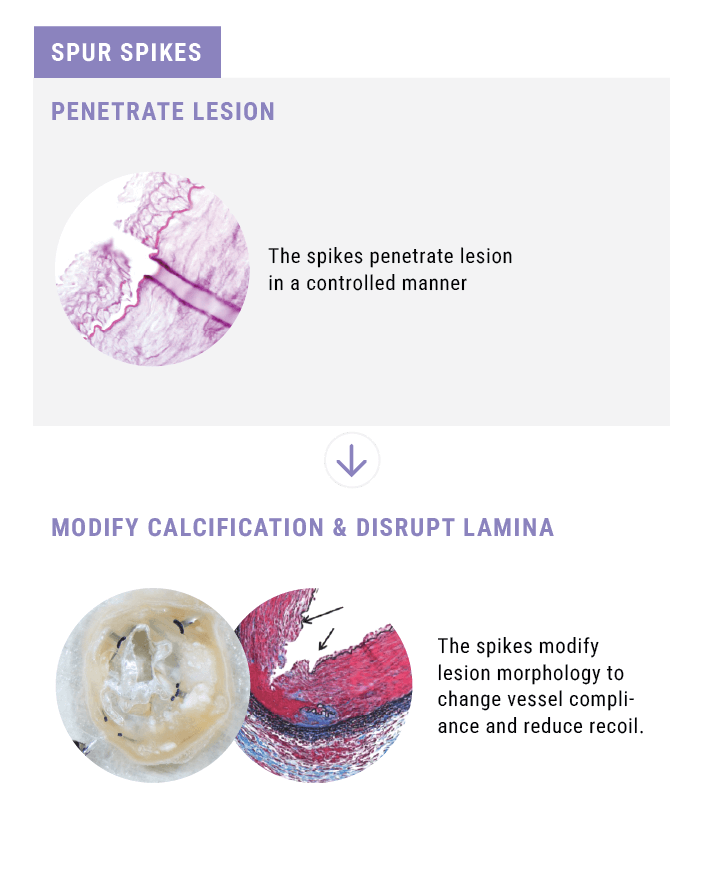
Spikes optimized for controlled penetration and lesion treatment
Integrated balloon system for improved conformability
Radiopaque markers for visibility
.014″ guidewire
Familiar pin-and-pull Spur deployment system
3.0mm and 4.0mm device diameters conform to vessel anatomy
Designed to treat long lesions, Spur can be utilized up to 4 times
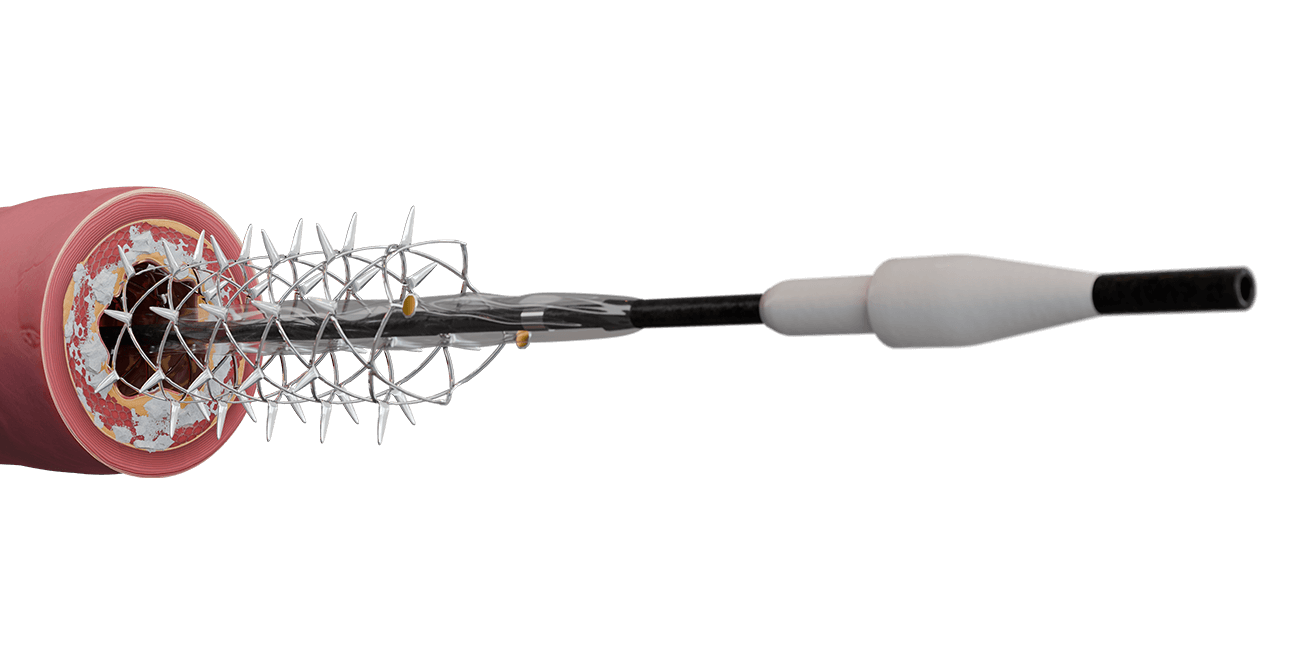
RST Mechanism of Action6
- Track Spur system to lesion site and deploy using pin-and-pull method.
- Inflate integrated balloon in a controlled fashion.
RADIAL SPIKES PENETRATE THE VESSEL WALL AND ARE DESIGNED TO:
- increase acute luminal diameter, and
- modify lesion morphology to change vessel compliance and reduce recoil.
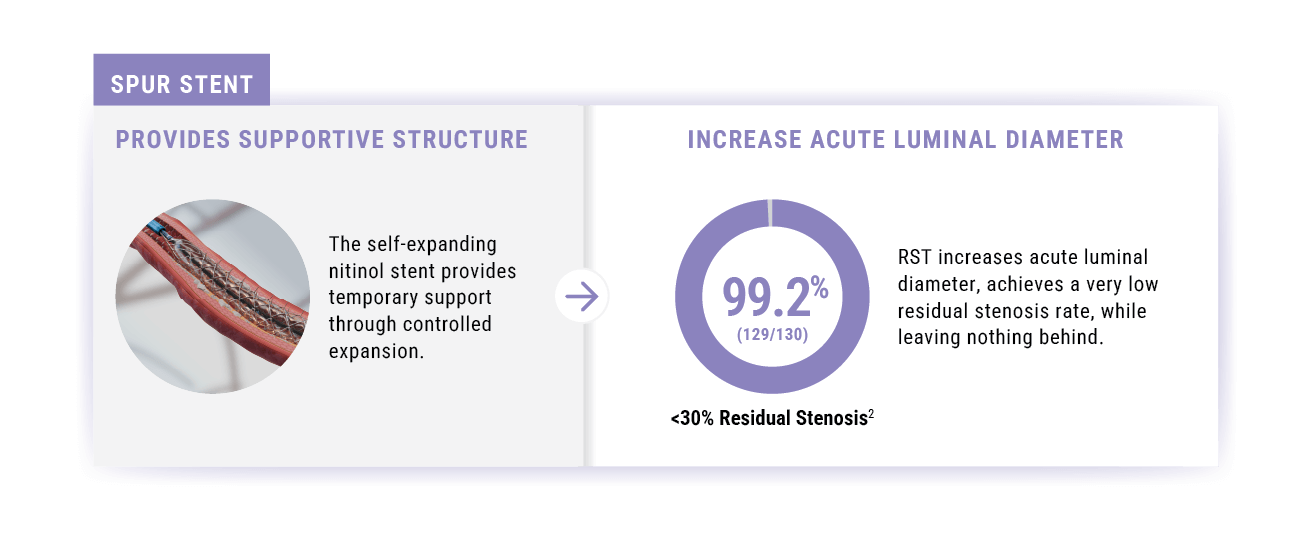
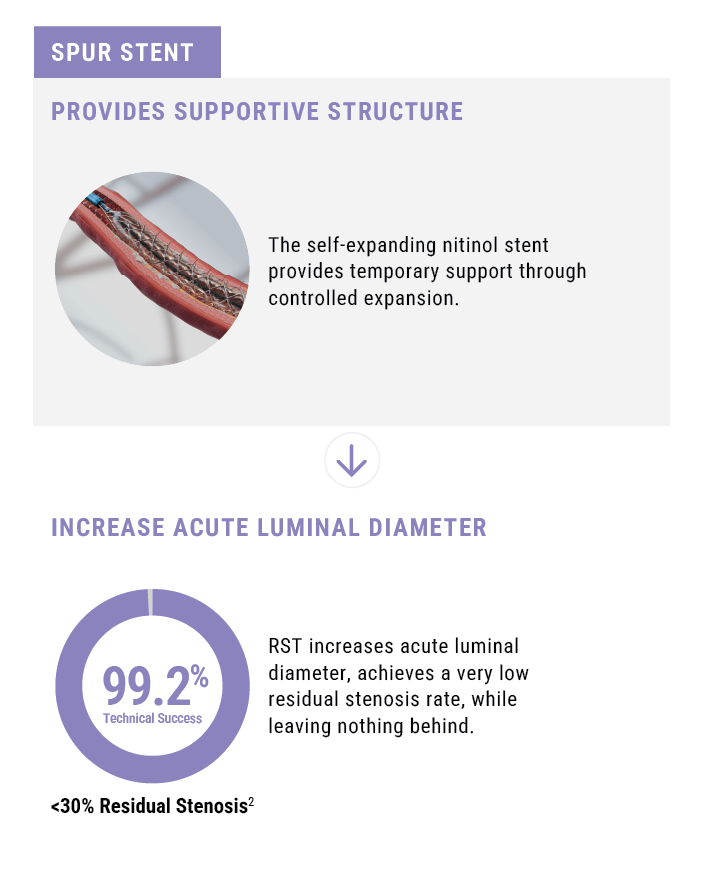
- Stent provides temporary supportive structure.
- Deflate balloon and reinflate balloon to fully expand the Spur.
- Recapture the Spur system.
FAMILIAR PIN-AND-PULL SPUR DEPLOYMENT SYSTEM

Clinically Proven
MAXIMIZING BTK* TREATMENT OUTCOMES WITHOUT COMPROMISING ON SAFETY
DEEPER REVEAL1
Prospective, multicenter, single-arm, performance goal comparator
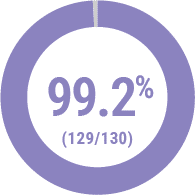
Technical Success2
(<30% Residual Stenosis)
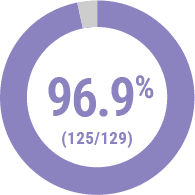
Freedom from MALE
and POD at 30 days3
STUDY DEVICE
Spur Peripheral Retrievable Stent System
STUDY SIZE
130 patients enrolled
49 centers (United States)
BASELINE CHARACTERISTICS
62.3% Rutherford class 5
37.7% Rutherford class 4
27% total occlusions
42% moderately/severely calcified lesions
96.4mm mean lesion length
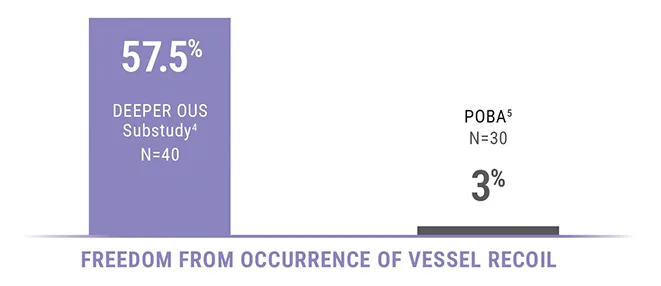
VESSEL RECOIL SUBSTUDY
Occurrence of vessel recoil is defined as lumen
compromise ≥10% at 15 minutes post Spur treatment
SPUR CASE EXPERIENCE
References
*Below the knee; 1. Data on file for DEEPER REVEAL clinical trial (NCT05358353); 2. Primary endpoint defined as Technical success: <30% residual stenosis by visual estimate within the treated lesion area on completion angiography. Core lab adjudicated technical success of <30% stenosis was 85.7% (120/140 lesions); 3. MALE defined as: above-the-ankle amputation of the index limb, OR major reintervention of the index limb involving the infrapopliteal arteries; Peri-operative death (POD); 4. Zeller et al. Early Tibial Vessel Recoil Following Treatment With the BareTemporary Spur Stent System: Results from the DEEPER OUS Vessel Recoil Substudy. Journal of Endovascular Therapy. 2024;0(0). doi:10.1177/15266028241280685; 5. Baumann et al. (2014). Early recoil after balloon angioplasty of tibial artery obstructions in patients with critical limb ischemia. Journal of Endovascular Therapy, 2014(21): 44–51; 6. Reflow Medical data on file.
Indications, Safety, & Warnings
CAUTION: This device is restricted to use by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the Indications, Contraindications, Warnings, Precautions, Adverse Events, and Procedural Steps.
Indications for Use
The Spur Peripheral Retrievable Stent System is intended as an adjunct to percutaneous transluminal angioplasty (PTA) to dilate stenoses in infrapopliteal arteries ranging in diameter from 2.5 mm to 4.5 mm.
Contraindications
The Spur is not intended for use in coronary and cerebral vasculature.
Warnings
Do not use the device past the expiration date on the label. Use of expired products may result in patient injury; Do not treat the target vessel more than four times with the same device. Do not deploy the Spur Stent System more than two times within the same vessel segment; Inspect the device packaging prior to use. Do not use the device if the device packaging has been damaged or if sterility has been compromised. Damaged product could result in patient injury; Use with caution in patients with a history of severe bleeding or coagulopathy; Ensure the Spur Stent System is used with appropriately sized ancillary devices as listed in the section below. Failure to do so could result in inadequate device performance or patient injury; Remove excess slack from the catheter (outside of the patient) to ensure the Spur Stent System is recaptured appropriately; If an inability to inflate or maintain balloon pressure occurs, remove the device and use a new one; Do not use excessive force or torque (more than 1 full turn) on the catheter as this could result in damage to the device and result in patient injury; This device contains nitinol, an alloy of nickel and titanium. Persons with allergic reactions to these materials may suffer an allergic reaction to this device. Prior to use, patients should be counseled on the materials contained in the device, as well as potential for allergy/hypersensitivity to these materials.
Precautions
This device should only be used by physicians experienced in interventional vascular procedures; The system is intended for single (one) use only. DO NOT re-sterilize and/or reuse; Inflate the balloon according to the balloon compliance chart. Balloon pressure should not exceed the rated burst pressure (RBP); Use only the recommended contrast medium to inflate the balloon to ensure adequate delivery; Perform all device manipulations under adequate fluoroscopy; Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met, determine the cause of the resistance before proceeding; Do not attempt to straighten a catheter if the shaft has become bent or kinked. Instead prepare a new catheter; During the procedure appropriate anticoagulant therapy must be provided to the patient as needed. Antiplatelet therapy should be prescribed post procedure in accordance with the treating physicians routine practice for endovascular procedures; Precautions should be taken when handling the device after exposure to patient, e.g. contact with blood. Used products are considered biohazardous material and should be disposed of properly as per hospital procedure; Ensure that predilatation achieves a lumen diameter greater than the outer diameter of the device catheter (approximately 2 mm) in order to advance the catheter.
Adverse Events
Additional intervention; Allergic reaction to drugs or contrast medium; Aneurysm or pseudoaneurysm; Hemorrhage, including bleeding at the puncture site; Inflammation; Occlusion; Pain or tenderness; Sepsis/Infection; Short term hemodynamic deterioration; Stroke; Death; Thrombosis; Vessel dissection, perforation, rupture, or spasm; Hematoma; Embolization; Pneumothorax or hemothorax; Shock; If the system is damaged, this product may perforate or dissect a blood vessel wall. Extreme caution needs to be taken when removing a damaged device. In the case of complications resulting from the removal of the entire system, stop procedure immediately, and perform appropriate treatment at the discretion of the physician.
©2025 Reflow Medical, Inc. All rights reserved. Reflow Medical, Spur and The Pulse of Medical Ingenuity are registered trademarks or trademarks of Reflow Medical, Inc. Federal law (USA) restricts these devices to sale by or on the order of a physician. Refer to the Instructions for Use for a complete listing of the Indications, Contraindications, Warnings, Precautions, Adverse Events, and Procedural Steps.
QUESTIONS?
TALK TO A REFLOW PRO.
For more information, please send us your question or request today. A Reflow pro will get back to you.